The Mantoux test is a qualitative, skin test to screen in vivo sensitization by Mycobacterium tuberculosis either due to active infection or past infection. It is also used to check the prophylaxis and efficacy of BCG vaccination. Mantoux test is a routine screening procedure for children, healthcare workers, individuals at high risk of being infected and individuals who are suspected of being infected with Tuberculosis.
Mantoux Test doesn’t distinguish between an active and a latent infection; nor does it provide a definitive diagnosis. If positive reaction occurs, additional tests such as sputum smear, culture and chest X-rays etc are necessary to establish a diagnosis of an active TB infection.
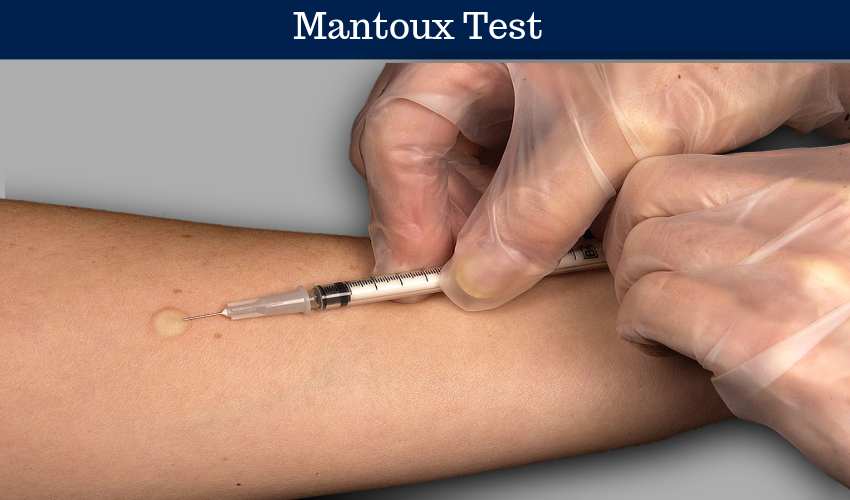
Principle of Mantoux Test
Mantoux test is based on a delayed type hypersensitivity reaction (Type IV) to test for individauls cell mediated immunity against Mycobacterium tuberculosis. Mycobacterial antigen is available in the form of Purified Protein Derivative (PPD). 5 units of PPD (0.1 ml) is injected intradermally with 26,27 or 30 gauze needle. The results are read in 48-72 hours for induration (elevated hardened area). Erythema (redness) is not significant.
After injection, cytokines are released by memory Th1 cells to attract macrophages and granulocytes which cause induration and erythema. Delayed hypersensitivity reaction begin after 5-6 hours and reach peak at 48-74 hours.
Requirements
- Graduated 1ml syringe
- PPD or Tuberculin
- Spirit swab
Procedure of Mantoux Test
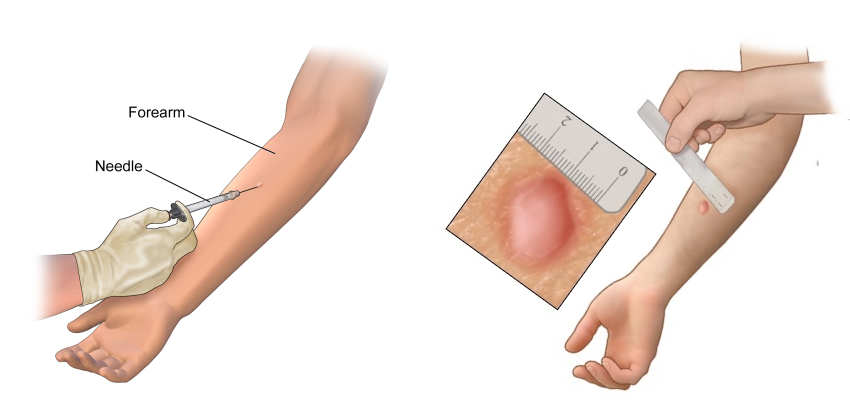
- Bring PPD reagent to room temperature.
- The preferred site for injection is dorsal surface of the forearm, about 4cm below the elbow joint. Select an area free of barriers (e.g. scars, sores).
- Disinfect the site of injection and allow to dry.
- Draw up just over 0.1 ml of PPD by using 1 ml syringe. Remove excess PPD to make exactly 0.1 ml and remove air from the syringe if present.
- Using 27 g needle to inject the PPD intradermally to make the deposition wheel, in the diameter of 6 to 8 mm which will rise up to the point of needle.
- Mark the area of injection with indicator.
- Read the result after 48-72 hours for induration.
Results and Interpretation
After 48-72 hours of administration of PPD, reaction should be measured in millimeters of induration (elevated hardened area). Erythema (redness) is not significant, it is thus not measured.
According to Center for Disease Control (CDC), interpretation of Mantoux test depends on two factors:
- Measurement in millimeters (mm) of the induration
- Person’s risk of being infected with TB and progression to disease if infected
Induration of ≥5 mm is considered positive in
- Human immunodeficiency virus (HIV)-infected persons
- Recent contacts of TB case patients
- Persons with fibrotic changes on chest radiograph consistent with prior TB
- Patients with organ transplants and other immunosuppressed patients
Induration of ≥10 mm is considered positive in
- Recent immigrants (i.e., within the last 5 years) from countries with a high prevalence of TB
- Injection drug users
- Residents and employees of the high-risk congregate settings like; prisons and jails, nursing, hospitals and other health care facilities, residential facilities for patients with AIDS and homeless shelters
- Mycobacteriology laboratory personnel
- Persons with the clinical conditions that place them at high risk; silicosis, diabetes mellitus, chronic renal failure, some hematologic disorders (e.g., leukemias and lymphomas), other specific malignancies (e.g., carcinoma of the head, neck, or lung)
- Infants, children, and adolescents exposed to adults at high risk for developing active TB
Induration of ≥15 mm is considered positive in
- Persons with no known risk factors for TB
Limitations of Mantoux Test
Mantoux Test doesn’t distinguish between an active and a latent infection. If positive reaction occurs, additional tests such as sputum smear, culture and chest X-rays etc are necessary to establish a diagnosis of an active TB infection.
Several factors can lead to false-positive or false-negative skin test reactions.
False Positive Reactions
Due to the test’s low specificity, most positive reactions in low-risk individuals are false positives. Some major causes of false positive Mantoux Test are:
- Infection with nontuberculous mycobacteria (NTM)
- BCG vaccination.
- Administration of incorrect antigen.
- Incorrect interpretataion of results.
False Negative Reactions
Some people have a negative reaction to the TST even though they have been infected with M. tuberculosis. A false-negative reaction can be caused by many things:
- Concurrent viral infection (e.g., measles, mumps, chicken pox, HIV)
- Concurrent bacterial infection (e.g., typhoid fever, brucellosis, typhus, leprosy, pertussis)
- Concurrent fungal infection
- Chronic renal failure
- Low protein states (e.g., severe protein depletion, afibrinogenemia)
- Diseases affecting lymphoid organs (e.g., Hodgkin’s disease, lymphoma, chronic leukemia, sarcoidosis)
- Immunosuppressive drugs (e.g., medical steroids)
- Children aged 6 months or less or elderly patients (i.e., immature or waning immunity)
- Stress (e.g., surgery, burns, mental illness, graft-versus-host reactions)
- Incorrect storage or handling of antigen or results that are not measured or interpreted properly
- Vaccinations using live virus; or
- Recent TB infection.

I really enjoyed all illustration of the Mantoux Test procedure.It enlighten me more about the test. Thanks.