- HbA: 96-98%, Composed of two alpha(α) and two beta(β) globin chains.
- HbA2: 1.5-3.5%, Composed of two alpha(α) and two delta(δ) globin chains.
- HbF: < 1.0%, Composed of two alpha(α) and two gamma(γ) globin chains.
Hemoglobin can combine with other substances, some normally and some abnormally and can also occur as:
- Oxyhemoglobin: Oxygen combined with hemoglobin.
- Carboxyhemoglobin: Carbon monoxide (CO) combined with hemoglobin.
- Carbaminohemoglobin: Carbon dioxide (CO2) combined with hemoglobin.
- Methemoglobin: Iron oxidized from its ferrous state to ferric state.
- Sulfhemoglobin: Sulfur combined with the hemoglobin.
- Cyanmethemoglobin: Methemoglobin bonded to cyanide ions.
Methods for Hemoglobin Estimation:
The different methods used for the estimation of hemoglobin can be divided as follows:
- Visual methods:
- Sahli’s method
- Dare method
- Haden method
- Wintrobe method
- Tallqvist method
- Spectrophotometric methods:
- Oxyhemoglobin method
- Cyanmethemoglobin method
- Gasometric method
- Automated hemoglobinometry
- Other methods:
- Alkaline-hematin method
- Specific gravity method
- Lovibond Comparator method
Cyanmethemoglobin method for hemoglobin estimation
The cyanmethemoglobin method or hemoglobincyanide method is the most accurate method of choice for the measurement of hemoglobin. It is a type of colorimetric/spectrophotometric method which has the following three major advantages over other methods:
- Measures all forms of hemoglobin except sulfhemoglobin, which is normally not present in the blood.
- Can be easily standardized, and
- Cyanmethemoglobin reagent is very stable.
Principle:
Blood is diluted 1:201 in a solution containing potassium ferricyanide (C6N6FeK3) and potassium cyanide (KCN). Potassium ferricyanide oxidizes hemoglobin in the sample to methemoglobin. The methemoglobin further reacts with potassium cyanide to form a stable-colored cyanmethemoglobin (hemiglobincyanide- HiCN) complex. The intensity of the colored complex is measured at 540 nm which is directly proportional to the amount of hemoglobin present in the specimen.
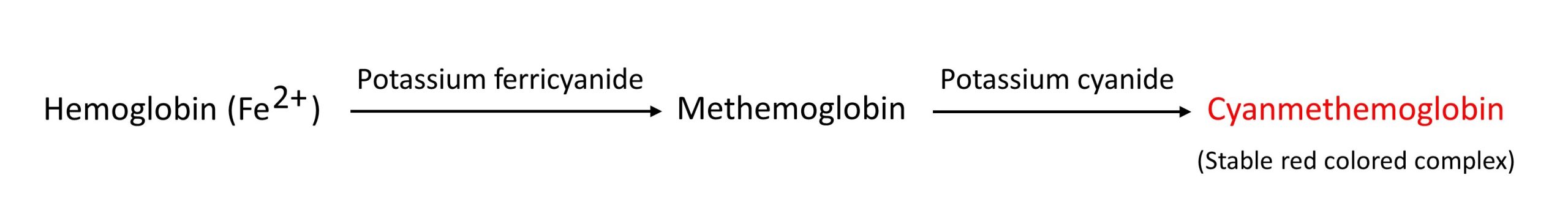
Requirements:
Specimen:
Capillary or venous blood. Venous blood should be anticoagulated with 1.5-1.8 mg EDTA per mL of blood and mixed immediately.
Reagents:
- Drabkin’s reagent, pH 7.0–7.4
The original cyanmethemoglobin technique was proposed by Stadie in 1920. This method used separated alkaline ferricyanide and cyanide reagents. A single reagent was introduced by Drabkin and Austin in 1935.This fluid contains:
- Potassium ferricyanide = 200 mg
- Potassium cyanide = 50 mg
- Potassium dihydrogen phosphate = 140 mg
- Non-ionic detergent = 1 ml
- Distilled or deionized water = to 1 liter
- Hemoglobin standard
Procedure:
- Label three clean, dry test tubes as Blank (B), Standard (S), and Test (T).
- Pipette as follows:
- Mix well and allow to stand at room temperature (250C) for 5 minutes.
- Measure the absorbance of the standard and test sample at 540 nm (green filter) against blank in a colorimeter. The color will be stable for up to several hours.
| Blank | Standard | Test | |
|---|---|---|---|
| Drabkin’s Reagent | 5 ml | 5 ml | 5 ml | Hemoglobin standard | – | 20 µl | – |
| Sample | – | – | 20 µl |
Calculation:
Calculate the concentration of hemoglobin in the specimen using the following formula:
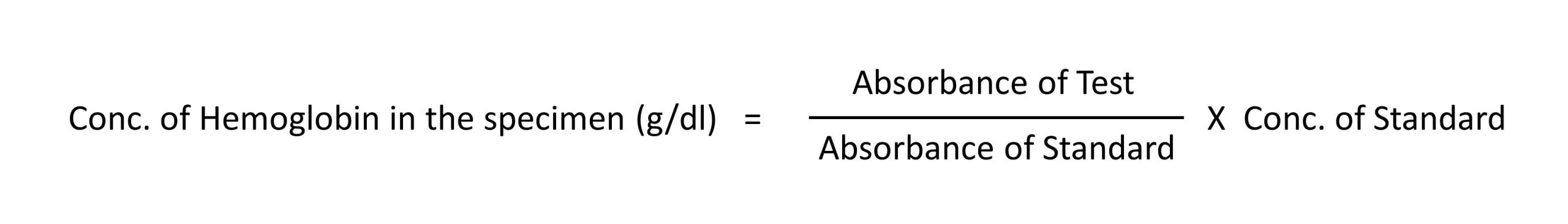
Alternatively, a calibration graph can be prepared using multiple standards of varying concentration and the result can be obtained quickly by checking hemoglobin concentration which corresponds to obtained absorbance. This is markedly acceptable when a large number of specimens are daily processed on the same instrument.
References:
- Pal, G.K., 2006. Textbook Of Practical Physiology-2Nd Edn. Orient Blackswan.
- DR .Sara Fadhil Bunea, Medical laboratory techniqes, Human physiology practical.
- Haemoglobin Reagent Product Insert – Tulip Diagnostics


Be the first to comment