Principle of Colorimeter :
When a beam of incident light of intensity I0 passes through a solution, following events occur:
- A part of incident light is reflected. It is denoted by Ir
- A part of incident light is absorbed. It is denoted by Ia
- Remaining incident light is transmitted. It is denoted by It
As Ir is kept constant by using cells with identical properties, The light that is not absorbed is transmitted through the solution and gives the solution its color. Note that color of the incident light should be complementary to that of color of the solution.
The ratio of the intensity of transmitted light (It) to the intensity of incident light (I0) is called transmittance (T). Photometric instruments measure transmittance. In mathematical terms,
T = It÷I0
The absorbance (A) of the solution (at a given wavelength) is defined as equal to the logarithm (base 10) of 1÷T. That is,
A = log (1÷T)
These measurements are dependent on two important laws:
-
Beer’s law:
When monochromatic light passes through a colored solution, the amount of light absorbed is directly proportional to the concentration (C) of solute in the solution.
-
Lambert’s law:
When monochromatic light passes through a colored solution, the amount of light absorbed is directly proportional to the length (L) or thickness of the solution.
When combining Beer-Lambert’s law,
Absorbance (A) α CL
Or, A= KCL
where K is a constant known as absorption coefficient.
As the path length is same (as same cuvette is used), Concentration of an unknown solution can be determined by using equation:

Instrumentation of Colorimeter :
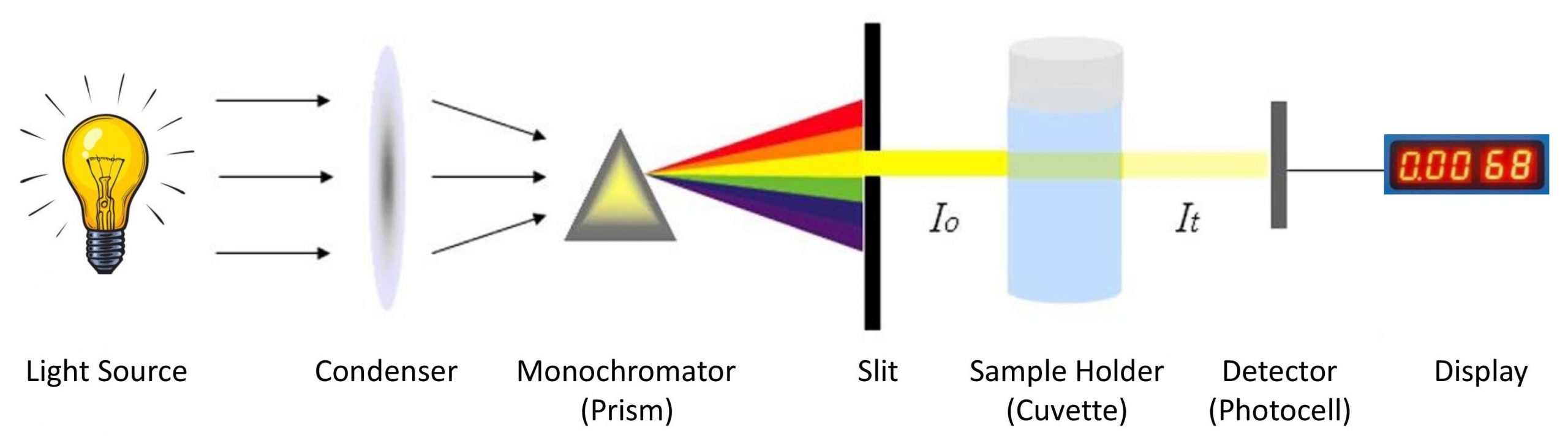
1. Light Source:
The light source should produce energy at sufficient intensity throughout the whole visible spectrum (380-780nm). Tungsten lamp is frequently used.
2. Slit:
It allows a beam of light to path and minimize unwanted light.
3. Condensing lens:
Give parallel beam of light.
4. Monochromator:
It is used to produce monochromatic radiation (one wavelength band) from polychromatic radiation (white light) produced from light source. It allows required wavelength to pass through it. Prism, gelatin fibers, grating monochromators or interference filters can be used.
5. Sample Holder (Cuvette):
Must be transparent. Glass or clear plastic cuvettes are preferred.
6. Photo detectors:
Detector of colorimeter basically receives the resultant light beam once it has passed through the sample and converts it into electrical signal. Selenium photocell, silicon photocell, phototube, photomultiplier tube etc are used.
7. Display:
It detects and measures the electric signal and makes visible output.
Uses and Applications
In clinical laboratory, colorimeter is used for the estimation of various biochemical compounds in variety of biological samples like blood, plasma, serum, CSF, urine and other body fluids. All those methods which involve the formation of colored product with specific analyte, the analyte can be estimated quantitatively. Colorimeters are also widely used for monitoring the growth of bacterial or yeast cells in liquid cultures.

Be the first to comment