When the rate of production exceeds, excess ketone bodies are eliminated in urine and the condition is known as ketonuria. Two conditions most commonly associated are starvation and Diabetes mellitus. Ketonuria is also seen in case of prolonged vomiting, severe diarrhoea, anesthesia, liver damage, high fat intake and low carbohydrate intake.
Various methods are available for detecting ketones in urine:
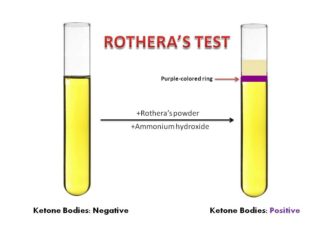
- Rothera’s test
- Gerhardt’s test
- Lang’s test
- Lindeman’s test
- Han’s test
- Tablet test
The commonly used methods for ketonuria are based on the principle of Rothera’s nitroprusside test. However, other tests with different principle can also be used. Gerhardt’s Test is not very sensitive test because it can only detect about 25 to 50 mg/dl of acetoacetic acid.
Principle of Gerhardt’s test
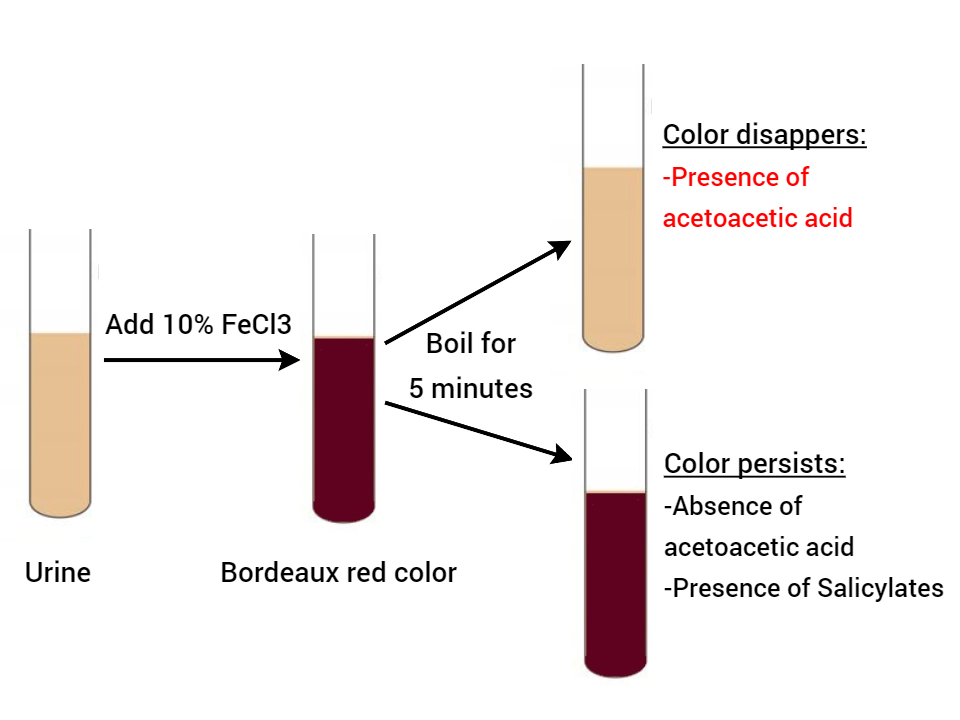
Gerhardt’s test is based on the reaction of ferric chloride with acetoacetic acid to form a port wine or Bordeaux red color. This test detects acetoacetic acid only, Acetone and beta-hydroxybutyrate can’t be detected by this method. It also detects salicylates in urine.
Requirements
- Urine specimen
- Test tubes
- 10% Ferric chloride:
(10ml of ferric chloride in 100 ml of distilled water)
Procedure of Gerhardt’s test
- Transfer about 3-5 ml of urine to a test tube.
- Add 5ml of ferric chloride drop by drop to the urine. If phosphates are present, they get precipitated as ferric phosphates.
- On addition of a slight excess of ferric chloride, if diacetic acid is present, Bordeaux red color will develop.
- To confirm the presence of acetoacetic acid, boil the test solution for 5 minutes.
- Observe the color change.
Observations and Results
- If the color disappears, then acetoacetic acid is present. The acetoacetic acid on boiling loses carbon dioxide and is converted to acetone. Acetone doesn’t react with ferric chloride.
- If the color persists, acetoacetic acid is absent. Previous color is due to salicylates.

Why is acetone can not react with acetoacetic acid