Methods for Creatinine Estimation
There are several methods available for the estimation of creatinine in blood. Some of the commonly used methods are as follows:
- Direct Chemical methods:
- Jaffe’s method
- Alkaline picrate (end point) or Modified Folin-Wu method
- Dinitrobenzene method (used in dry chemistry)
- Indirect Enzymatic methods:
- Deaminase method (one enzyme step method)
- Creatininase method (multi-enzymatic method)
- Other (Gold standard) methods:
- High-performance liquid chromatography
- Gas chromatography with mass spectrometry
Jaffe’s method for Creatinine estimation
Jaffe’s method is widely employed in clinical laboratories as a rapid and cost-effective colorimetric technique for measuring creatinine levels in serum and urine. It is highly non-specific. Substances like glucose, protein, ketones, and certain drugs (e.g., cephalosporins) can react with the picrate, causing falsely elevated creatinine readings by 15–25%. It requires deproteinization, typically using tungstic acid (sodium tungstate and sulfuric acid) before the reaction.
Principle:
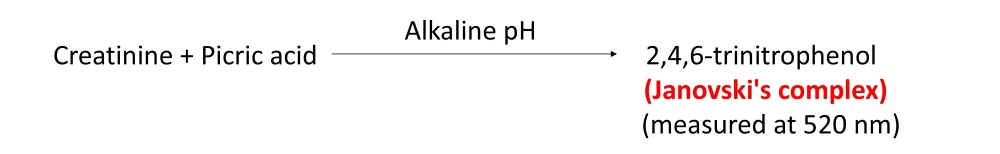
Creatinine present in a protein-free filtrate of blood or serum reacts with picric acid in an alkaline medium to form an orange-red colored complex known as creatinine picrate (Janovski’s complex or 2,4,6-trinitrophenol). The intensity of the color produced is directly proportional to the concentration of creatinine in the sample. This colored complex is measured spectrophotometrically at a wavelength of approximately 520–540 nm using a green filter.
Requirements
Specimen
- Serum or heparinized plasma
Creatinine stability: 24 hours at 2-8°C - Urine: dilute sample 1/50 with distilled water.
Creatinine stability: 1 day at 2-8°C.
Multiply results by 50 (dilution factor).
Reagents
- Sodium tungstate (10%)
- Sulphuric acid (2/3N)
- Picric acid (0.04 M)
- Sodium hydroxide (0.75 N)
- Creatinine standard (4 mg/dl or 0.04 mg/ml)
Instruments
- Test tubes
- Pipettes, disposable tips, rack
- Water bath
- Colorimeter
Procedure:
A. Preparation of Protein-free filtrate
- Label three clean, dry test tubes as Blank (B), Standard (S), and Test (T).
- Pipette as follows:
- Mix the contents after each addition. Keep the tubes at room temperature for 10 minutes.
- Filter the Test (T) tube and collect the filtrate.
| Blank | Standard | Test | |
|---|---|---|---|
| Distilled water | 4 ml | 3 ml | 3 ml |
| Standard | – | 1 ml | – |
| Serum | – | – | 1 ml |
| Sodium tungstate | 2 ml | 2 ml | 2 ml |
| Sulphuric acid (2/3 N) | 2 ml | 2 ml | 2 ml |
B. Estimation of Creatinine
- Prepare another set of three tubes and label as Blank (B), Standard (S), and Test (T).
- Pipette as follows:
- Mix and keep the test tubes at room temperature for 15 minutes.
- Measure the absorbance of the standard and test sample at 520-540nm (green filter) against blank.
| Blank | Standard | Test | |
|---|---|---|---|
| Picric acid | 1 ml | 1 ml | 1 ml |
| Sodium hydroxide | 1 ml | 1 ml | 1 ml |
| Distilled water | 3 ml | – | – |
| Standard filtrate | – | 3 ml | – |
| Test filtrate | – | – | 3 ml |
Calculation:
For preparing protein-free filtrate, a 1:4 dilution was done (2 ml of blood in a total volume of 8 ml). However, only 3 ml of the protein-free filtrate was used, which is equivalent to 0.75 ml of the blood sample. Standard contains 0.04 mg/ml, which is diluted to 8 ml; therefore, 3 ml will have 0.015 mg. Hence, we can calculate the concentration of serum creatinine in the specimen using the following formula:
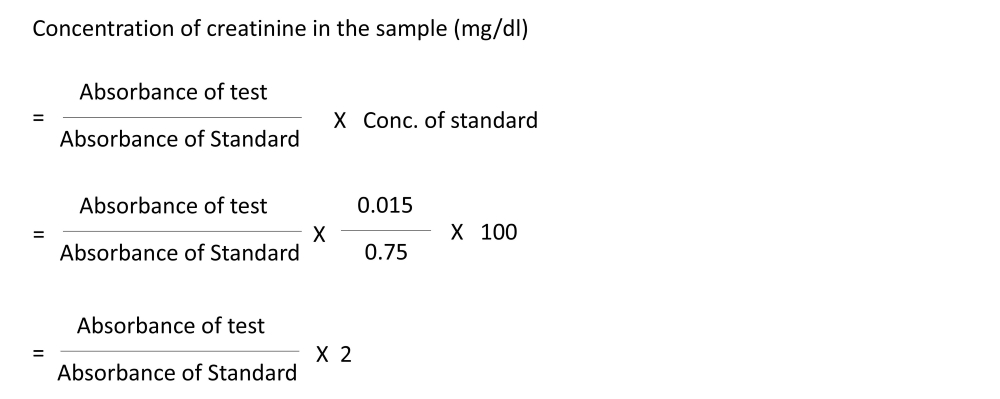
Reference range
| SI unit | Conventional unit | Conversion factor | |
|---|---|---|---|
| Adult male | 53-106 µmol/L | 0.5-1.1 mg/dl | mg/dl*88.4 = µmol/L |
| Adult female | 44-97 µmol/L | 0.5-1.1 mg/dl | |
| Elderly | May be low | May be low |
Result interpretation and Clinical significance
Increased serum creatinine levels indicate:
- Renal causes
- Acute and chronic renal failure
- Glomerulonephritis and nephritis
- Diabetic and hypertensive nephropathy
- Drug-induced renal impairment
- Renal malignancy
- End-stage renal disease (ESRD)
- Post-renal causes (urinary obstruction)
- Prostatic hypertrophy
- Ureteric or bladder stones
- Urethral stricture
- Tumors of urinary bladder or ureter
- Extra-mural urinary obstruction
- Other conditions
- Severe or vigorous exercise
- Muscle injury, muscle breakdown, muscular dystrophy
- Reduced renal blood flow (dehydration, shock, heart failure)
- Pre-eclampsia and eclampsia
- Hepatorenal syndrome
- Drug intake (e.g., aminoglycosides, cephalosporins, cimetidine, trimethoprim)
- Physiological variation
- Slight increase with advancing age (related to body mass)
Decreased serum creatinine levels are seen in:
- Low muscle mass or small body stature
- Muscle atrophy
- Myasthenia gravis
Advantages and Limitations
Advantages
- Simple, rapid and easy to perform
- Cost-effective and economical
- Suitable for routine clinical laboratory use
- Can be easily automated
Limitations
- Non-specific reaction – other substances also react with alkaline picrate
- Positive interference from glucose, ketone bodies, proteins, ascorbic acid, and bilirubin
- Interference by drugs such as cephalosporins
- Overestimation of serum creatinine values
- Less accurate at low creatinine concentrations
- Affected by hemolysis and lipemia
- Lower specificity compared to enzymatic methods
Modifications
Several modifications of Jaffe’s method have been developed to reduce analytical interference and improve the accuracy of serum creatinine estimation.
| Modification | Principle / Key Feature | Main Advantage | Limitation |
|---|---|---|---|
| Folin–Wu Jaffe’s Method | Uses protein-free filtrate before Jaffe reaction | Reduces protein interference | Time-consuming; still non-specific |
| Lloyd’s Reagent Method | Adsorption of creatinine on Fuller’s earth | Improves specificity | Extra handling steps required |
| Modified (Kinetic) Jaffe’s Method | Measures rate of color development | Reduces interference from non-creatinine chromogens | Some interference still persists |
| Rate-Blanked Jaffe’s Method | Uses sample blank to correct non-specific reactions | Better accuracy than classical Jaffe | Not completely specific |
| Compensated Jaffe’s Method | Mathematical correction for known interferences | Improved agreement with enzymatic methods | May vary between analyzers |
References
- BASU P. BIOCHEMISTRY LABORATORY MANUAL: FOR MBBS, BDS, BHMS, BAMS, BUMS, BNYS AND DMLT STUDENTS. Academic Publishers; 2016.
- Mohanty B, Basu S. Fundamentals of practical clinical biochemistry.
- Dandekar SP, Rane SA. Practicals and Viva in Medical Biochemistry. New Delhi: Elsevier; 2004.
- Vasudevan DM, Das SK. Practical Textbook of Biochemistry for Medical Students. Jaypee Brothers Medical Publishers; 2013.
- Sood R. Concise book of Medical Laboratory Technology. Jaypee Brothers Pvt. Limited; 2015.

Be the first to comment